

| Origin: |
|
The major clones are defined by concurrent differences in mtDNA, ploidy level and allozymes and differ consistently in dorsal coloration pattern, which allows them to be identified in the field. Minor clones are nested in major clones and differ from one another by slight variations in allozymes. The nesting of minor clones within major clones suggest that they diverged within a major clone via mutation (Bolger / Case, 1994). It is considered that the diploid clonal lineages were derived from hybridizations between bisexual species. The triploid clonal lineages possibly originated through back crosses between the diploid clones and males of parental species (Cole et al., 1988; Darevsky, 1992; Moritz, 1983; Radtkey et al., 1995; Volobouev et al., 1993; Sources: Ota et al., 1989; Ota et al., 1996). It took a long time until the sexual ancestors involved in hybridization had been identified. In 1995 studies of Radtkey et al. revealed that Lepidodactylus lugubris is a cross between Lepidodactylus moestus (described by Ota et al., 1995) and an other undescribed species (Lepidodactylus sp.) from Arno Atoll (Marshall Islands) and that independent clone production through hybridization of these is probaby recent, ongoing and has occurred several times. 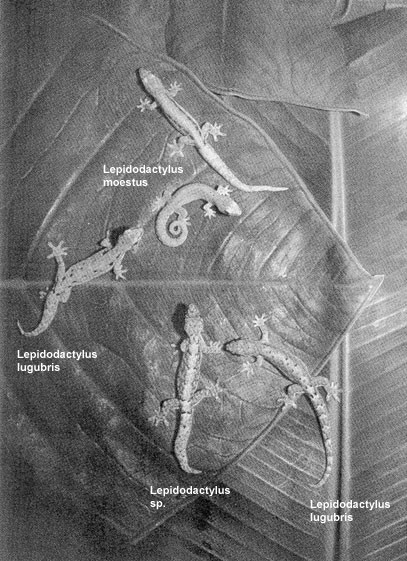
Two parthenogenetic clones Lepidodactylus lugubris with the two parental species. Two colour morphs of the maternal ancestor Lepidodactylus moestus are shown in the upper middle, the undescribed paternal ancestor is in the lower middle and two Lepidodactylus lugubris clones are middle-left and lower-right. Back pattern polymorphisms of the maternal species carry over into the Lepidodactylus lugubris clones. Photograph by J. Newsome. Source: Figure 1, page 147 of Radtkey, R.R., S.C. Donellan, R.N. Fisher, C. Moritz, K.A. Hanley & T.J. Case, 1995. When species collide: the origin and spread of an asexual species of gecko. Proc. R. Soc. Lond. B259: 145-152 Boissinot et al. (1997) also assumed that the parthenogens originated on Arno Atoll where Lepidodactylus moestus and Lepidodactylus sp. coexist. Their investigations (1997) in French Polynesia (Moorea Island, Maiao Island, Society Archipelago, Rangiroa Atoll, Takapoto Atoll, Tuamoto Archipelago) showed that the bisexual species of Takapoto is not the parental population of the local clones, but may well belong to the parental species. Boissinot et al. (1997) suggest that the origin of clone A is independend from that of clone B, C and D and that clone B and C could have originated from hybridization between diploid clone D and males of different bisexual populations. Moritz et al. (1993) indicate independent origins of the diploid clones 2nA, 2nB-I and 2nB-II. They assumed that triploid clone 3nB possibly arose by fertilization of diploid 2nB by males of sexual species and a similar event involving a diploid 2nA female resulted in the triploid 3nA clone. The variety of different lineages in Lepidodactylus lugubris still is not well studied yet. Here descriptions of some lineages (Ineich, 1987/1988; Ineich / Ota 1992; Yamashiro / Toda / Ota, 2000): |
| Some Clones: | >> top of page |
|
The lineages are determined with reference to the descriptions of
Ineich (1988).
Diploid clone A (Takapoto Atoll): Two longitudinal rows of six to eight (mostly seven) dark dots from the neck to the base of the tail, dorsal ground color light gray or ivory (Ineich, 1988; Ineich / Ota 1992) Triploid clone B (Takapoto Atoll): Numerous dark spots, especially enlarged and mostly paired on the neck and the base of the tail. Dorsal ground color light gray or ivory (Ineich, 1988; Ineich / Ota, 1992) Triploid clone C (Takapoto Atoll): Two longitudinal rows of six to eight dark dots from the neck to the base of the tail, two longitudinal thin stripes on the neck, dorsal ground color often reddish brown (Ineich, 1988; Ineich / Ota 1992) Clone E, ploidy unknown (Takapoto Atoll): No fixed dark patterns, dorsal ground color reddish brown (Ineich, 1988; Ineich / Ota, 1992) Diploid Clone D (Moorea Island): Less than 7 spots restricted to the anterior medio-dorsal band. (Ineich, 1988) Diploid Clone A (Haha-jima, Ogasawara Islands): Seven or eight pairs of small v-shaped markings along the middorsal line only (Yamashiro / Toda / Ota, 2000) Triploid Clone B (Kitadaitojima, Daito Islands): Lateral black bars on the neck and base of tail, w-shaped mark on head, two dorsolateral rows of bold crescent-shaped black spots on body (Yamashiro / Toda / Ota, 2000) Triploid Clone C (Zamami-jima / Okinawa Islands,Ryukyu Archipelago): Dorsolateral black bars on the neck and base of tail and indistinct w-shaped marks along the middorsal line (Yamashiro / Toda / Ota, 2000) Diploid Clone Da (Minamidaito-jima, Daito Islands): Dorsal pattern of lateral black bars on the neck and base tail, chevrons and short bars alternating with each other along the middorsal line, a relatively distinct w-shaped mark on the head (Yamashiro / Toda / Ota, 2000). |
 Diploid Clone A (Haha-jima, Ogasawara Islands) |
 Triploid Clone C (Zamami-jima / Okinawa Islands, Ryuku Archipelago) |
Photos of: 'Clonal composition of the Parthenoge- netic Gecko, Lepidodactylus lugubris, at the Northernmost Extremity of Its Range', Saiko Yamashiro, Mamoru Toda and Hidetoshi Ota, 2000 |
 Diploid Clone Da (Minamidaito-jima, Daito Islands) |
 Triploid Clone B (Kitadaitojima, Daito Inseln) |
 Triploid Clone B' (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-1 (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-2 (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-3 (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-4 (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-5 (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-6 (Minamidaito-jima, Daito Islands) |
 Triploider Clone BI-7 (Minamidaito-jima, Daito Islands) |
 Triploid Clone BI-8 (Kitadaito-jima, Daito Islands) |
 Triploid Clone N (Minamidaito-jima, Daito Islands) |
|
Diploid Group Clone 2nA (Fiji/Hawaii/Western Pacific): Except for the clone 2nA-III from Hawaii A back pattern (Ineich, 1988), regular paired mid-dorsal chevron markings. Clone 2nA-III on Hawaii has additional marks on the side of the neck (Moritz et al., 1993). Diploid Group Clone 2nB-I (Fiji): Clone B back pattern (Ineich, 1988) with strong lateral markings on the neck and irregular dorsal markings (Moritz et al., 1993). Diploid Group Clone 2nB-II (Tahiti): back pattern resembling the clone D back pattern (Ineich, 1988) with the small numbers of mid-dorsal markings, except that the markings are posterior rather than anterior (Moritz et al., 1993). Triploid Group Clone 3nA (Samoa): Clone A back pattern (Ineich, 1988; Moritz et al., 1993). Triploid Group Clone 3nB: Variable, sometimes clone B-like back pattern (Ineich, 1988; Moritz et al., 1993). |
| Hybrides: | >> top of page |
|
Often crosses between clones and bisexual males result in hybrides with degenerated
reproductive gonads.
These animals may show female or male characteristics.
Studies of Ivan Ineich and Hidetoshi Ota (1992) on Takapoto Atoll revealed that
hybrides often were significantly bigger than bisexual Lepidodactylus sp.
(at that time still also described as Lepidodactylus lugubris) or
Lepidodactylus lugubris clones.
The sterile hybrides studied by Boissinot et al. (1997) on Takapoto Atoll all were determined to be the result of mating between diploid clone A and males of the local bisexual Lepidodactylus sp.. No hybrides issued from fertiliation of females belonging to clone B, C or D were found. Boissinot et al. (1997) were not sure if this can be explained by the lower frequency of these clones or genetic or chomosomal incompatibilities between them and the bisexuals or if there are other reasons. |
| Clones & Males: | >> top of page |
|
If no males of other species are involved, usually hatchlings of eggs laid by parthenogenetic females are also parthenogenetic females.
So, in many reports about "male" Lepidodactylus lugubris later turned out that the specimen was a male of a closely related Lepidodactylus species or a hybrid. But there are exceptions. Rösler (1992) reported two well developped males, which hatched under terrarium conditions from eggs of Lepidodactylus lugubris. Unfortunately he did not indicate if the males were fertile. As the origin of the mother geckos could not be determined, he was not sure if one of the two females was bisexual, but assumed that both were parthenogenetic and males hatched for uncleared reasons. Orlando Cuellar and Arnold G. Kluge (1972) reported that the seemingly anomalous shape and surface texture of the testes in most of the Lepidodactylus lugubris-"males" suggests that spermatogenesis could not have proceeded mormally. Furthermore in the case of Lepidodactylus lugubris there are some circumstantial data indicating that the males may not be of hybrid origin. In the first place hybrid individuals usually exhibit phenotypes deviating from the normal condition of either parent species. The males that Cuellar and Kluge found were not obviously different from the typical female Lepidodactylus lugubris phenotype. 1998 Yamashiro and Ota described a sterile "male" of Lepidodactylus lugubris from Ishigakijima Island (Yaeyama Group, Ryukyu Archipelago) which looked the same as the local clone C females and also because of a lack of related species could not have been a product of hybridization. They assumed that the "male" had developped from a normal clone C because of hormonal changes. Among the 673 Lepidodactylus lugubris examined by Cuellar and Kluge (1972) on Oahu, Hawaii only 4 individuals showed male characteristics. Radtkey et al. (1995) even indicate only 5 "males" among over 8000 geckos on Hawaiian Islands and Marianas Islands. Now one of my own juveniles also seems to develop to a "male". This specimen hatched from an egg which was incubated at temperatures of sometimes around 30° C during summer. I don´t know if the high incubation temperature effected development to a male or if other factors were the reason. |

|
|
Susan G. Brown and Susan Murphy-Walker (1996) reported the interactive behaviour between a sterile but well devolpped male ("male phenotype") and one of six females in reproductive stages each: The male was more likely to approach females with no sign of egg development or small eggs than females with large eggs and was observed neck-biting and moving on top of females although no copulations were observed. Eventually either the male or the females did not possess the behavioural repertoire needed to respond to and complete a normal courtship and mating sequence. In studies of Brown et al. (1993) in groups consisting offemales only interactive behaviour decreased over time, reflecting a stabilization of the dominance relationships between the females. High levels of interactive behaviour were maintained between male / female dyads and females showed more aggressive behaviour than males. As in other gecko species none of the females responded to the males multiple chirp call, which most likely functions as a territorial spacing mechanism repelling other males (Marcellini, 1977b). |

|
|
My male gecko is mostly ignored by the females. Often he approaches a female with the "multiple chirp call" described by Brown et al. (1993), which sounds like a series of calls, each not unlike the single call
of the females.
A several times I saw him climbing over and below a female and thereafter repeatedly neckbiting and releasing. The female remained unimpressed during this procedure and after a while left the exited male alone. |
| >> top of page |